| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2006-05-22 15:12:47 UTC |
|---|
| Update Date | 2023-02-21 17:16:35 UTC |
|---|
| HMDB ID | HMDB0003288 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Selenocysteine |
|---|
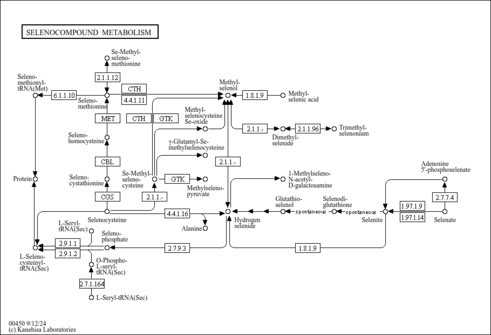
| Description | Selenocysteine is an amino acid that is present in several enzymes (for example glutathione peroxidases, tetraiodothyronine 5' deiodinases, thioredoxin reductases, formate dehydrogenases, glycine reductases and some hydrogenases). Selenocysteine has a structure similar to cysteine, but with an atom of selenium taking the place of the usual sulfur. Proteins that include a selenocysteine residue are called selenoproteins (Wikipedia ). Selenocysteine is a naturally occurring amino acid in both eukaryotic and prokaryotic organisms. It is found in tRNAs and in the catalytic site of some enzymes. The genes for glutathione peroxidase and formate dehydrogenase contain the TGA codon, which codes for this amino acid (Pubchem). |
|---|
| Structure | InChI=1S/C3H7NO2Se/c4-2(1-7)3(5)6/h2,7H,1,4H2,(H,5,6)/t2-/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Selenyl-L-alanine | ChEBI | | L-Selenocystein | ChEBI | | L-Selenozystein | ChEBI | | (2R)-2-Amino-3-selanylpropanoate | HMDB | | (2R)-2-Amino-3-selanylpropanoic acid | HMDB | | 3-Seleno-alanine | HMDB | | 3-Selenoalanine | HMDB | | L-Selenocysteine | HMDB |
|
|---|
| Chemical Formula | C3H7NO2Se |
|---|
| Average Molecular Weight | 168.05 |
|---|
| Monoisotopic Molecular Weight | 168.964200301 |
|---|
| IUPAC Name | (2R)-2-amino-3-selanylpropanoic acid |
|---|
| Traditional Name | L-selenocysteine |
|---|
| CAS Registry Number | 3614-08-2 |
|---|
| SMILES | N[C@@H](C[SeH])C(O)=O |
|---|
| InChI Identifier | InChI=1S/C3H7NO2Se/c4-2(1-7)3(5)6/h2,7H,1,4H2,(H,5,6)/t2-/m0/s1 |
|---|
| InChI Key | ZKZBPNGNEQAJSX-REOHCLBHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - L-alpha-amino acid
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Amine
- Organic oxide
- Hydrocarbon derivative
- Organopnictogen compound
- Selenol
- Primary amine
- Organoselenium compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Primary aliphatic amine
- Organic nitrogen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Selenocysteine,1TMS,isomer #1 | C[Si](C)(C)OC(=O)[C@@H](N)C[SeH] | 1277.4 | Semi standard non polar | 33892256 | | Selenocysteine,1TMS,isomer #2 | C[Si](C)(C)N[C@@H](C[SeH])C(=O)O | 1392.9 | Semi standard non polar | 33892256 | | Selenocysteine,2TMS,isomer #1 | C[Si](C)(C)N[C@@H](C[SeH])C(=O)O[Si](C)(C)C | 1425.8 | Semi standard non polar | 33892256 | | Selenocysteine,2TMS,isomer #1 | C[Si](C)(C)N[C@@H](C[SeH])C(=O)O[Si](C)(C)C | 1279.7 | Standard non polar | 33892256 | | Selenocysteine,2TMS,isomer #1 | C[Si](C)(C)N[C@@H](C[SeH])C(=O)O[Si](C)(C)C | 1434.6 | Standard polar | 33892256 | | Selenocysteine,2TMS,isomer #2 | C[Si](C)(C)N([C@@H](C[SeH])C(=O)O)[Si](C)(C)C | 1534.0 | Semi standard non polar | 33892256 | | Selenocysteine,2TMS,isomer #2 | C[Si](C)(C)N([C@@H](C[SeH])C(=O)O)[Si](C)(C)C | 1353.7 | Standard non polar | 33892256 | | Selenocysteine,2TMS,isomer #2 | C[Si](C)(C)N([C@@H](C[SeH])C(=O)O)[Si](C)(C)C | 1579.8 | Standard polar | 33892256 | | Selenocysteine,3TMS,isomer #1 | C[Si](C)(C)OC(=O)[C@H](C[SeH])N([Si](C)(C)C)[Si](C)(C)C | 1540.0 | Semi standard non polar | 33892256 | | Selenocysteine,3TMS,isomer #1 | C[Si](C)(C)OC(=O)[C@H](C[SeH])N([Si](C)(C)C)[Si](C)(C)C | 1424.7 | Standard non polar | 33892256 | | Selenocysteine,3TMS,isomer #1 | C[Si](C)(C)OC(=O)[C@H](C[SeH])N([Si](C)(C)C)[Si](C)(C)C | 1385.0 | Standard polar | 33892256 | | Selenocysteine,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)[C@@H](N)C[SeH] | 1536.3 | Semi standard non polar | 33892256 | | Selenocysteine,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N[C@@H](C[SeH])C(=O)O | 1650.8 | Semi standard non polar | 33892256 | | Selenocysteine,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N[C@@H](C[SeH])C(=O)O[Si](C)(C)C(C)(C)C | 1894.6 | Semi standard non polar | 33892256 | | Selenocysteine,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N[C@@H](C[SeH])C(=O)O[Si](C)(C)C(C)(C)C | 1698.6 | Standard non polar | 33892256 | | Selenocysteine,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N[C@@H](C[SeH])C(=O)O[Si](C)(C)C(C)(C)C | 1689.7 | Standard polar | 33892256 | | Selenocysteine,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N([C@@H](C[SeH])C(=O)O)[Si](C)(C)C(C)(C)C | 2014.9 | Semi standard non polar | 33892256 | | Selenocysteine,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N([C@@H](C[SeH])C(=O)O)[Si](C)(C)C(C)(C)C | 1768.3 | Standard non polar | 33892256 | | Selenocysteine,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N([C@@H](C[SeH])C(=O)O)[Si](C)(C)C(C)(C)C | 1759.0 | Standard polar | 33892256 | | Selenocysteine,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](C[SeH])N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2252.4 | Semi standard non polar | 33892256 | | Selenocysteine,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](C[SeH])N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2039.3 | Standard non polar | 33892256 | | Selenocysteine,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](C[SeH])N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 1793.4 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Selenocysteine GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-5900000000-827b47dc191649f521ac | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Selenocysteine GC-MS (1 TMS) - 70eV, Positive | splash10-00di-5900000000-32e999fe6e3d67e322cb | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Selenocysteine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Selenocysteine 10V, Positive-QTOF | splash10-01b9-0900000000-fadd5a69b2b225d034ac | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Selenocysteine 20V, Positive-QTOF | splash10-01b9-0900000000-8fe6412cebc1ca99fe59 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Selenocysteine 40V, Positive-QTOF | splash10-00dl-5900000000-2408222e037ff9db752a | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Selenocysteine 10V, Negative-QTOF | splash10-014i-0900000000-91ba91d891f89ff1fc23 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Selenocysteine 20V, Negative-QTOF | splash10-00kk-6900000000-67c579b8ba30e155bcb3 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Selenocysteine 40V, Negative-QTOF | splash10-00di-9200000000-0b58b77f13cec3df121d | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Selenocysteine 10V, Negative-QTOF | splash10-014i-0900000000-59cfe0ab1984ab09d7af | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Selenocysteine 20V, Negative-QTOF | splash10-01b9-7900000000-6f634fdb5ee7a8437ca7 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Selenocysteine 40V, Negative-QTOF | splash10-014i-1900000000-0f58b4784272be073705 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Selenocysteine 10V, Positive-QTOF | splash10-01b9-0900000000-3a288e1fc4c42cfb333c | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Selenocysteine 20V, Positive-QTOF | splash10-05fr-0900000000-c29c42f6924485632ad3 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Selenocysteine 40V, Positive-QTOF | splash10-0a4i-2900000000-8f03b287aede9d710058 | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| General References | - Chu FF, Esworthy RS, Doroshow JH, Doan K, Liu XF: Expression of plasma glutathione peroxidase in human liver in addition to kidney, heart, lung, and breast in humans and rodents. Blood. 1992 Jun 15;79(12):3233-8. [PubMed:1339300 ]
- Mostert V, Wolff S, Dreher I, Kohrle J, Abel J: Identification of an element within the promoter of human selenoprotein P responsive to transforming growth factor-beta. Eur J Biochem. 2001 Dec;268(23):6176-81. [PubMed:11733012 ]
- Zimmermann MB, Kohrle J: The impact of iron and selenium deficiencies on iodine and thyroid metabolism: biochemistry and relevance to public health. Thyroid. 2002 Oct;12(10):867-78. [PubMed:12487769 ]
- Sun QA, Su D, Novoselov SV, Carlson BA, Hatfield DL, Gladyshev VN: Reaction mechanism and regulation of mammalian thioredoxin/glutathione reductase. Biochemistry. 2005 Nov 8;44(44):14528-37. [PubMed:16262253 ]
- Blotcky AJ, Ebrahim A, Rack EP: Determination of selenium metabolites in biological fluids using instrumental and molecular neutron activation analysis. Anal Chem. 1988 Dec 15;60(24):2734-7. [PubMed:3245598 ]
- Utomo A, Jiang X, Furuta S, Yun J, Levin DS, Wang YC, Desai KV, Green JE, Chen PL, Lee WH: Identification of a novel putative non-selenocysteine containing phospholipid hydroperoxide glutathione peroxidase (NPGPx) essential for alleviating oxidative stress generated from polyunsaturated fatty acids in breast cancer cells. J Biol Chem. 2004 Oct 15;279(42):43522-9. Epub 2004 Aug 4. [PubMed:15294905 ]
- Rooseboom M, Vermeulen NP, Andreadou I, Commandeur JN: Evaluation of the kinetics of beta-elimination reactions of selenocysteine Se-conjugates in human renal cytosol: possible implications for the use as kidney selective prodrugs. J Pharmacol Exp Ther. 2000 Aug;294(2):762-9. [PubMed:10900258 ]
- Zinoni F, Birkmann A, Stadtman TC, Bock A: Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4650-4. [PubMed:2941757 ]
|
|---|